Differing Event-Related Patterns of Gamma Band Power in Brain Waves of Fast and Slow Reacting Subjects
Download a .pdf version of this paper (1186k)
Hennric Jokeit
Institute for Medical Psychology, University of Munich, Goethestrasse 31,
80336 Munich, Germany
and
Scott Makeig
Naval Health Research Center, PO Box 85122, San Diego, CA 92186-5122
Current address: Computational Neurobiology Laboratory, The Salk Institute,
La Jolla CA, scott@salk.edu
Abstract
Fast- and slow-reacting subjects exhibit different patterns of circa 40 Hz band electroencephalographic (EEG) activity when responding as quickly as possible to auditory stimuli. This result appears to confirm long-standing speculations of Wundt that fast- and slow-reacting subjects produce speeded reactions in different ways, and demonstrates that analysis of event-related changes in the amplitude of EEG activity recorded from the human scalp can reveal information about event-related brain processes unavailable using event-related potential (ERP) measures. Time-varying spectral power in a selected (35 to 43 Hz) gamma frequency band was averaged across trials in two experimental conditions: passive listening and speeded reacting to binaural clicks, forming 40 Hz event-related spectral responses. Factor analysis of between-subject event-realted spectral response differences split subjects into two near-equal groups comprised of faster and slower-reacting subjects respectively. In faster-reacting subjects, 40 Hz power peaked near 200 and 400 ms poststimulus in the react condition, whereas in slower-reacting subjects, 40 Hz power just before stimulus delivery was larger in the react condition. These group differences were preserved in separate averages of relatively long and short reaction-time epochs for each group. Gamma band (20-60 Hz) filtered ERP response averages did not differ between the two groups or conditions. Because of this, and since gamma band power in the auditory ERP is small compared to the EEG, the observed ERS-response features must represent gamma band EEG activity reliably induced by, but not phase-locked to experimental stimuli or events.
Introduction
Nearly a century ago, Wilhelm Wundt proposed that there are two types of subjects in simple RT experiments: fast-reacting subjects, who respond before they fully perceive the stimulus, and slower-reacting subjects who wait for a more complete stimulus perception before making a response (1). Although anatomical and physiological studies have demonstrated extensive interconnections within brain sensory and motor systems that might enable equivalent motor output to be produced via activity in different neural pathways (2,3), qualitative subject differences in electrophysiological processing during RT tasks have not yet been identified.
Two different approaches are available for analyzing EEG dynamics during event-related response experiments. Time-domain response averages, termed event-related potentials (ERPs), isolate potential deviations that appear in successive trials at the same time and in the same phase or polarity relative to an experimental event. By contrast, averages of time-varying event-related spectral (ERS) power reveal event-related modulations of ongoing or stimulus-induced oscillatory EEG activity that are roughly time-locked, but not specifically phase-locked to such events (4). The ways in which ERPs change when subjects actively respond to auditory stimuli instead of passively listening to them are well known (5). But while it is known that mean EG spectral power in several frequency bands covaries with changes over time in performance of simple tasks (6,7), less is known about rapid event-related changes in non-phase-locked EEG activity during task performance (8).
This is particularly true for gamma band EEG frequencies (25 to 90 Hz or higher) that are most commonly supposed to be associated with awareness or conscious perception (9-14). In human subjects, gamma band activity is enhanced during intense vigilance and performance of cognitive tasks (6,9-10,15-17), suppressed during central anesthesia and slow wave sleep (11,12), and has been proposed to play essential roles in olfactory recognition, temporal integration, visual feature binding and segregation, and sensorimotor integration (18-24). Human ERPs evoked by auditory and other stimuli contain some gamma band oscillations (25-27), but in animal cortex, gamma band activity induced by olfactory and visual stimuli usually appear as irregular bursts roughly time-locked, but not phase-locked to stimulus onsets (20,28-31).
The present study answers three questions: (1) How does the EEG frequency spectrum after presentation of brief auditory stimuli differ when subjects react quickly to the stimuli instead of passively listening to them? (2) Do gamma band components of the stimulus-locked ERP also differ in the two conditions? (3) Do fast- and slow-reacting subjects have qualitatively similar or different dynamic patterns of gamma band EEG activity during the response task? Our analysis of time-varying power in the gamma frequency band demonstrates that two different modes of auditory response processing underlie between-subject RT differences, supporting claims that gamma band activity has functional significance in sensorimotor processing.
Methods
Twenty-three right-handed adults (ages 20-53) were tested in two conditions. First, 110 clicks (2 ms wide square pulses, 75 dB SPL) were presented binaurally through headphones at random inter-stimulus intervals of 3 to 7 s. Next, subjects were asked to react as quickly as possible to a second set of 275 identical clicks by pressing a response button with the right index finger. EEG epochs of 640 ms beginning 128 ms before each click were recorded with a sampling rate of 2000 Hz using a 12 bit A/D converter with an analog high pass filter cutoff of 0.67 Hz and a 50 Hz notch filter to exclude line frequency artifacts. The single-channel EEG montage (Cz referred to linked mastoids) was chosen to maximize the chances of capturing event-related gamma band activity in auditory and sensorimotor cortices (9,16,25). RT was recorded separately with a temporal resolution of 1 ms. Trials with RTs shorter than 100 ms or longer than 600 ms were rejected from the analysis. To exclude large eye movements and muscle activity, epochs in which potential anywhere exceeded +/-70 uV were also eliminated. On average, 80% of the trials were analyzed.
After data collection, response epochs were low pass filtered with a 100 Hz cutoff and downsampled to a sampling rate of 250 Hz to minimize computer processing. All filtering used symmetric Butterworth filters with 24 dB/octave slopes. Downsampled epochs were then averaged for each subject and condition, creating wide band ERPs. Averaged evoked gamma band responses (GBRs) were calculated separately by applying a 20 to 60 Hz bandpass filter to epochs before averaging. Mean event-related power spectra were computed by applying a fast Fourier transform (FFT) to a Hanning-tapered (-35, 500 ms) data window from each wide band (1-100 Hz) response epoch, extracting power at each frequency, and averaging. Individual subject and grand mean spectra were calculated in both task conditions.
To compute time-varying 40 Hz-band event-related spectral (ERS) responses, each 20-60 Hz pre-filtered response epoch was divided into 68 overlapping 24-point (96 ms) time windows with a shift interval of 8 ms. After tapering with a Gaussian window and zero-padding to 64 points, each window was converted to spectral power using an FFT. Since gamma band power was affected by the 50 Hz notch filter used in the recording, the range 35 to 43 Hz was chosen for analysis. Power in this range was integrated for each time window using a Hamming function. The resulting 40-Hz ERS-response transform for each epoch thus consisted of 68 power estimates at 8 ms intervals. Statistical significance of response differences in the various measures was tested by repeated measures analysis of variance, by Bonferroni-corrected t-tests for dependent samples, and by t-tests for independent samples, using p<.01 as threshold of significance.
Results

|
Fig. 1. Responses to clicks in passive listening (noRT) and reaction time (RT) task conditions. Grand means of responses from 23 adult subjects (site Cz, band pass 1-100 Hz with notch filtering at 50 Hz). (A,B) Event-related potential (ERP) waveforms. Abscissa is time re the onset of the click. The ordinate is potential in uV. Note the larger positive peak near 300 ms (P300), and the smaller positive peak near 200 ms (P200) in the RT condition. (C) Difference wave between waveforms in traces A and B. (D,E) Grand mean gamma band responses (GBRs), event-related potentials (from traces A and B) band pass filtered between 20 and 60 Hz. (F) Difference wave between GBRs in D and E. (G,H) Grand mean response epoch power spectral transforms of wide band (1-100 Hz) filtered response epochs (-30, 500 ms) with analog notch filtering at 50 Hz. Abscissa shows EEG frequency; ordinate, EEG power in uV 2 . (I) Difference spectrum between the traces in G and H. Note the reduction in power near 20 Hz and the increase above 30 Hz in the RT condition. Power near 50 Hz is reduced by line filtering.
As expected, the wide band (1-100 Hz) grand mean ERP in the speeded response (RT) condition (Fig. 1A) contains three prominent auditory response components, N100, P200 and P300, whereas in the passive listening (noRT) condition, the P300 is absent (Fig. 1B). The grand mean difference wave between the RT and noRT conditions (Fig. 1C) reflects the significantly smaller P200, and larger P300 components in the RT condition (5). Both RT and noRT grand mean 20-60 Hz GBRs (Fig. 1D,E) contain excursions of up to +/- 1.5 uV during the first 100 ms after click presentation but do not vary as a function of task (p=0.622)(Fig. 1F). However, the grand mean response spectra for the two tasks (Fig. 1G,H), and their difference (Fig. 1I), reveal that response epochs contain more power above 30 Hz and less power near 20 Hz in the RT than in the noRT condition. Although analog line filtering used during data collection obscures task differences near 50 Hz, between 35 and 43 Hz mean spectral power is significantly higher in the RT condition. A task difference is also present at frequencies above 50 Hz, suggesting that in humans stimulus-induced gamma band activity may appear as wide band bursts similar to those seen in animals (19).
To determine whether event-related 40-Hz dynamics varied with mean RT, ERS responses in the RT condition were averaged separately for subgroups of five subjects with fastest (167+/-36 ms) and slowest (272+/-57 ms) mean RTs. These two subgroup ERS-response means were then used as templates to split the remaining subjects into two groups. First, each subject's ERS response in the RT condition was correlated with both subgroup response templates, and then was assigned to the group that had a response template with which it was more positively correlated. For one subject, both correlation coefficients were negative, and that subject was rejected from further analysis. In this manner, ten subjects were assigned to the fast responder (Fast) group, and 12 were assigned to the slow responder (Slow) group. When the grand mean ERS responses of the enlarged groups were used as the correlation templates, no subject assignment changed. The splitting procedure also separated subjects by mean RT. No subject in the Slow responder group had a lower mean RT than any of the Fast responder group, and accordingly, the mean RT of the 10 Fast responders (176+/-44 ms) was significantly shorter than that of the 12 Slow responders (244+/-78 ms).
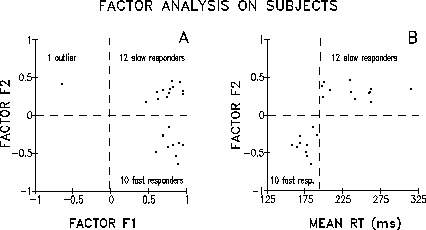
|
Fig. 2. Factor analysis on subjects (Q-Factor Analysis) was performed on a Pearson correlation matrix on task differences between individual event-related spectral (ERS) responses near 40 Hz. (See text and Fig. 3 for details). Output submitted to Varimax rotation. (A) Scatter plot of the subjects factor loading on the second factor (F2) against their loading on the first factor (F1). With the exception of one outlier (left), all subjects load positively on the F1. However, F2 splits the sample by sign of loading (+/-). (B) Scatter plot of F2 against individual mean RT. Abscissa: mean RT in ms. Ordinate: the subject's loading on F2. Note that F2 separates subjects by mean RT: no subject in the Slow responding group had a lower mean RT than any of the Fast group.
As a check of the implication that 22 subjects' ERS-response pattern differences formed two distinct groups, the Pearson correlation matrix for the 23 subjects' ERS-response differences (RT-noRT) was submitted to a Q-factor analysis on subjects with Varimax rotation. With one exception, the same subject whose response correlated negatively to both response templates (Fig. 2a, left), all subjects loaded positively on the first factor (F1) (Fig. 2A), which explained 46% of the total variance; the outlier subject was widely separated from the rest of the group. The second factor (F2), explaining 25% of variance, clearly split the other 22 subjects into two groups by its (+/-) sign of loading (Fig 2A). Plotting the 22 individual F2 loadings against individual mean RTs (Fig. 2B), two subject groups differing both in gamma band dynamics and performance clearly emerge. The ten subjects whose mean RTs were shorter than 195 ms load in the opposite direction to the twelve remaining subjects whose mean RTs were longer than 195 ms. The sign of loading on F2 reproduces exactly the group affiliations determined by template correlation.
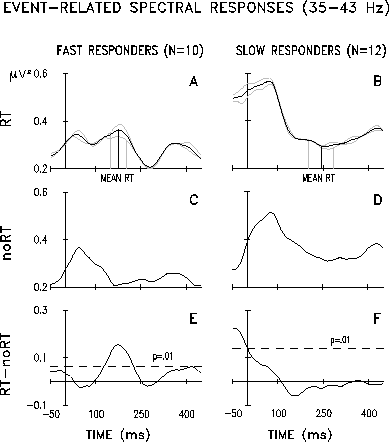
|
Fig. 3 Grand mean event-related spectral (ERS) responses for two subject subgroups: 10 Fast responders and 12 Slow responders from the original 23 subjects. Task conditions: reaction time (RT) and passive listening (noRT). Each trace plots mean time-varying power at 40 Hz at site Cz (referred to linked mastoids). See text for details of group selection and spectral transform parameters. Abscissa: time re stimulus onset. Ordinate: power in uV 2 . (A) Mean ERS responses of 10 Fast responders and (B) Mean ERS responses of 12 Slow responders, both in the RT task. The dotted traces show the ERS-response averages for short-RT and long-RT epochs (relative to subject median) from the Fast and Slow responders, respectively. The solid vertical lines represent grand mean RTs, and the dotted vertical lines, the means of above- and below-median RTs, respectively. (C) Mean ERS responses of 10 Fast responders and (D) 12 Slow responders in the noRT task condition. (E) Difference wave between the RT and noRT condition responses shown in A and C. (F) Difference wave between the traces shown in B and D. Dotted lines in D and E show limits of significant difference (p<.01).:
Grand mean ERPs, GBRs, mean spectra, and 40-Hz ERS responses were then calculated separately for the two subject groups. There were no significant group differences in the mean spectra (p=0.992), nor in the GBRs (p=0.999), and except for P300 amplitude, which was negatively correlated with RT, there were no differences in the two groups' wide band ERPs. Figures 3A and 3B show grand mean 40-Hz ERS responses for the Fast and Slow responder groups in the RT condition. Although mean ERS response power did not differ significantly between the two groups (p=0.282), its temporal dynamics were significant across groups, as was the interaction of group and dynamics. Results for the noRT condition (Fig. 3C,D) were similar: group difference in mean ERS-response power was not significant (p=0.192), but its temporal dynamics were significant, and interacted with group affiliation. Latency of the first poststimulus ERS-response peak (Fig. 3A-D) was near-significantly (p=0.025) longer (76+/-27 ms) in Slow responders than in Fast responders (53+/-19 ms).
Difference waves between ERS responses in RT and noRT conditions for the two groups (Fig. 3E,F) show the effects of task and group affiliation on 40-Hz dynamics. The most prominent difference wave components for Fast responders are peaks near 200 and 400 ms that appear only in the RT condition (Fig. 3A). In contrast, the task ERS response difference for Slow responders (Fig. 3F) contains no peaks after stimulus presentation. Instead, these subjects have significantly larger mean 40-Hz power just before the stimulus in the RT condition (Fig. 3B) than during passive listening (Fig. 3D).
To test the consistency of the group ERS-response differences, for each subject epochs in the RT condition were grouped into relatively short-RT and long-RT subsets, depending on whether RT was shorter or longer than the subject median. Dotted lines in Figs. 3A and 3B show ERS-response averages for the long-RT and short-RT epochs of each group. Between-group ERS-response differences are clearly maintained in this split-half comparison, even between ERS responses from long-RT epochs of Fast responders (RT=201+/-48 ms) and from short-RT epochs of Slow responders (RT=202+/-36 ms), data subsets that do not differ significantly in mean RT (p=0.536). Statistical comparison confirmed the absence of a significant effect of RT subset on ERS-response dynamics (p=0.574).
Discussion
Results of our ERS-response analysis support three conclusions: First, two stable but qualitatively different patterns of event-related changes in EEG power near 40 Hz occur during production of simple speeded reactions in different subjects. Second, the two response patterns divide subjects into two roughly equal groups. Third, the two patterns are associated with different mean RTs, one produced by relatively quick responders, and the other produced by subjects who on average react more slowly. The results demonstrate that equivalent mean EEG power can result from different time-varying spectral patterns. In the reaction time condition, slower responders' vertex EEG contains relatively larger 40-Hz power before the stimulus; after the stimulus, there is no difference between reacting and passive task conditions (Fig. 3F). In contrast, faster responders have equally low 40-Hz power before the stimulus in both conditions, but their ERS responses in the reaction time task contain phasic relative increases in 40-Hz power near 200 and 400 ms poststimulus (Fig. 3A,E). Because significant 40-Hz activity in the ERP does not last longer than 120 ms (Figs. 1D-F), the later ERS response peaks in fast responders must reflect gamma band activity induced by, but not phase-locked to stimulus presentations and/or motor responses, and therefore not appearing in the ERP.
A plausible interpretation of the faster-reacting subjects' ERS-response patterns is that during the RT task, auditory stimuli induce a series of modulations of the probability of appearance of stimulus-induced cortical gamma band bursts or transients. Event-related enhancements of gamma band activity with similar latencies have also been found in two quite different studies. Enhancements of the auditory steady-state response (SSR) driven by clicks or tones repeating at rates near 40 Hz also peak near 200 and 400 ms under some conditions (32), and a half-cycle jerk of a visual grating induces gamma band oscillatory activity in cat striate cortex that peaks near 200 ms (33). It is not known whether there are similar group differences in EEG modulation patterns after imperative visual stimuli, but it is possible that the similar time courses of event-related modulations of spontaneous, of stimulus-induced, and of driven gamma band activity reflect the action of a common brain modulatory system or systems involved in attention and production of speeded responses.
Several central ascending transmitter systems are known to modulate the abundance of auditory cortical response activity (16,34). In particular, stimulation of the nucleus basalis of Meynart can rapidly induce onset of high-frequency oscillations in the auditory cortex of rats, suggesting that stimulus-induced activation of ascending cholinergic outflow from the nucleus basalis, stimulated by input from the reticular formation following attended, task-relevant auditory stimuli (35), may enhance stimulus-induced gamma band oscillations in auditory cortex of fast responders in the RT task. The nucleus basalis is also involved in producing the contingent negative variation (CNV), a low-frequency potential appearing before expected events (36), suggesting that cholinergic activation might also be responsible for slow responders' larger pre-stimulus gamma band activity in the RT task. Although the observed ERS-response pattern differences appear compatible with this or other central modulatory models, our data do not allow definite conclusions about generators of response features.
Two recent reports of behavioral experiments involving auditory choice RTs have proposed grouping subjects into fast and slow responders on the basis of differences in their RT histograms (37,38). Neither those authors nor we have detected a basis for a group difference in stimulus-locked ERPs, implying that important aspects of performance-related brain processing are represented in changes in phase-incoherent, but not in phase-coherent gamma band activity. To what extent does ERP activity contribute to ERS responses? Probably very little, because although the 20-60 Hz filtered GBR contains potential deviations larger than 1 uV (Fig. 1D, 1E), peak 35-43 Hz power in the GBR amounts to 0.03 uV 2 , a small fraction of ERS-response power at the same moment (70 ms). In general, for all frequencies above about 10 Hz, ERS-response means are little affected by adding or removing ERP activity, because at these frequencies the ratio between power in the (phase-incoherent) EEG and (phase-coherent) ERP is large (4).
Group differences in response times have been modeled as differences in response bias (35), in accord with the hypothesis of Wundt and others that slower ("sensorial") responders wait to fully perceive a stimulus and then react to their perception, whereas the process of fast ("muscular") responding was described by Kuelpe as involving, "a somewhat indistinct sensation of the initiating stimulus [which often] does not become clear until the reaction has been performed" (39). This hypothesis, that response initiation in faster responders may precede some aspects of stimulus perception, is compatible with our finding that RT differences between relatively fast- and slow-reacting subjects arise from group differences in neurophysiological processing during response preparation and execution. The significant interaction of subject group and ERS-response dynamics in the passive listening condition, appearing to arise from a near-significant group difference in the mean latency of the early poststimulus peak in gamma band power, also suggests the presence of early auditory-processing differences between the two groups. The stability across RT subsets of the 300 ms ERS-response minimum and 400 ms maximum for Fast responders (Fig. 3A) shows that the two groups differ in brain processes occurring after the motor response as well, possibly associated with differences in information integration or consolidation.
Do the two groups only produce different proportions of relatively short and long RTs and associated ERS responses, or does only one ERS-response pattern characterize all or most of each subject's responses? The high within-group consistency of ERS-response means for short-RT and long-RT epochs (Fig. 3A,B dotted lines) implies that differences between the two groups' response patterns do not arise directly from timing differences in executing motor responses. Rather, the consistency of group responses differences in these split-half comparisons suggests that a large majority of most subjects' ERS response patterns are correlated with a single ERS-response template.
The larger pre-stimulus 40-Hz power for Slow responders in the RT condition (Fig. 3B,F) was not accompanied by a group difference in pre-stimulus power at 20 Hz. The relative decrease in 20 Hz EEG power in the RT condition (Fig. 1I) may be a consequence of active suppression of a focal brain rhythm at or near 19 Hz generated in or near the primary somatomotor areas in both humans and animals during quiet vigilance and motor preparation, that is suppressed before voluntary movements (3,15,40,41).
Do stimulus- or response-linked muscle potentials contaminate the ERS response results? This possibility can be reasonably rejected for the most part, because (a) the ERS-response patterns do not correspond to known latencies of auditory stimulus-evoked muscle activity (42), (b) ERS-response power near 250 ms after clicks is equal in both task conditions for the Slow responder group (Fig. 3F), and (c) short-RT versus long-RT epoch comparisons within both subject groups (Fig. 3A,B dotted lines) contain no ERS-response features that differ in latency. It is unlikely, therefore, that muscle potentials significantly contaminate the poststimulus ERS records. However, we cannot rule out the possibility that the higher pre-stimulus activity during the react condition in slow responders might in part arise from a difference in anticipatory muscle activity.
The late 40-Hz ERS-response peak near 400 ms in Fast responders (Fig. 3A) might arise from a peak in 34 to 42-Hz activation recently shown to be generated over the motor cortex about 200 ms after voluntary finger movements (43). Gamma band EEG has long been associated with states of high arousal, alertness, or attention (6,10,15,16). The pre-stimulus 40-Hz group difference, therefore, might instead reflect slower responders' stronger anticipation of imperative stimuli (14), or a more focused preparation to react to them (25). Their more intense anticipation might, in turn, require more fully elaborated stimulus processing, accompanied by a more distinct perception of the stimulus, to initiate a motor response, as Wundt and other early psychologists suggested, resulting in slower mean RTs.
It is now well-established that ERP techniques provide convenient, noninvasive measures of phase-coherent event-related brain activity (23). The present results show that wide- and narrow-band spectral response-averaging methods provide parallel noninvasive measures of the dynamics of phase-incoherent event-related brain processes (4), and demonstrate that stable group differences in neurophysiological processing can be detected in EEG recordings, even during simple tasks performed at equivalent levels of performance. Further experiments are required to study the topographic distribution of ERS-response patterns at all frequencies, to determine the relationship between ERS responses and low-frequency ERP features, to compare subject behavioral and response characteristics on simple and choice reaction tasks, and to characterize the behavioral concomitants of the observed ERS-response differences. Finding qualitative group differences in psychophysiological processing during speeded responding, as opposed to graded individual differences in response speed, also suggests the possibility of using time-frequency averaging methods to identify neurophysiological concomitants of other subject and task differences (44,45).
We thank Peter Bartsch and his colleagues at the department of Neurophysiology (Charite, Berlin) for the opportunity to perform the experiments and Ulla Mitzdorf and Robert Galambos for suggestions on the manuscript. This research was supported by Deutsches Forshungsgemeinschaft (Jo 242/1-1, Po 121/17-1), by 'Friedrich-Baur-Stiftung'. Dr. Makeig's collaboration was supported by the Naval Medical Research and Development Command project 62233N.3P30.0008-6308.
Key terms: 40 Hz EEG / oscillations / event-related potentials /
reaction times / time-varying spectra / event-related spectra
Abbreviations: electroencephalogram (EEG), reaction time (RT),
event-related potential (ERP), event-related spectral (ERS) response,
gamma band response (GBR), steady-state response (SSR).
Disclaimer. This work was supported in part by the Department of the Navy, Naval Medical Research and Development Command, Bethesda, Maryland under work unit ONR.WR.30020 (6429).The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government. Approved for release, distribution unlimited.
Communicated by Robert Galambos.
References
1. Wundt, W. (1913) Grundriss der Psychologie (Engelmann, Leipzig: Germany).
2. Schiller, P. H., Sandell, J. H. & Maunsell, R. (1987) J Neurophysiol 57, 1033-1049.
3. Houk, J. C., Keifer, J. A. & Barto, G. (1993) Trends in Neurosci 16, 27-33.
4. Makeig, S. (1993) Electroencephalogr clin Neurophysiol 86, 283-293.
5. Picton, T. W., Hillyard, S. A., Krausz, H. I. & Galambos, R. (1974) Electroencephalogr clin Neurophysiol 36, 179-190.
6. Makeig, S. and Inlow, M. (1993) Electroencephalogr clin Neurophysiol , 86:23-35.
7. Belyavin, A. and Wright, N.A. (1987) Electroencephalogr clin Neurophysiol , 66:137-144.
8. Pfurtscheller, G. (1977) Electroencephalogr clin Neurophysiol 43, 757-750.
9. Krieger, D. & Dillbeck, M. (1987) Electroencephalogr clin Neurophysiol 67, 222-230.
10. Sheer, D. E. (1989) in Brain Dynamics: Progress and Perspectives , eds. Basar, E. & Bullock, T. H. (Springer-Verlag, Berlin) pp. 339-374.
11. Madler, C. & Poppel, E. (1987) Naturwissenschaften 74, 42-43.
12. Llinas, R. & Ribary, U. (1993) Proc Natl Acad Sci USA 90, 2078-81.
13. Basar, E., Gonder, A. & Ungan, P. (1976) Biol Cybern 25, 27-40.
14. Crick, F. & Koch. C. (1990) Semin Neurosci 2, 263-275.
15. Boyer, J. J., Montaron, M. F., Vahne, J. M., Albert, M. P. & Rougeul, A. (1987) Neuroscience (Oxford) 22, 863-869.
16. Steriade, M., Dossi, R. C., Par, D. & Oakson, G. (1991) Proc Natl Acad Sci USA 88, 4396-4400.
17. Freeman, W. J. & van Dijk, B. W. (1987) Brain Res 422, 267-276.
18. Freeman, W. (1975) Mass Action in the Nervous System (Academic Press, New York).
19. Gray, C. M. & Singer, W. (1989) Proc Natl Acad Sci USA 86, 1698-1702.
20. Eckhorn, R., Bauer, R., Jordan, W., Brosch, M., Kruse, W., Munk, M. & Reitboeck, H. J. (1988) Biol Cybern 60, 121-130.
21. Kristofferson, A. B. (1984) Ann N.Y. Acad Sci 423, 3-15.
22. Engel, A. K., Konig, P. & Singer, W. (1991) Proc Natl Acad Sci USA 88, 9136-9140.
23. Murthy, V. N. & Fetz, E. (1992) Proc Natl Acad Sci USA 89, 5670-5674.
24. Poppel, E. (1978) in Handbook of Sensory Physiology , eds. Held, R., Leibowitz, H. W. & Teuber, H.-L. (Springer, Berlin), vol. VIII: Perception, pp. 713-729.
25. Galambos, R., Makeig, S. & Talmachoff, P. (1981) Proc Natl Acad Sci USA 78, 2643-2647.
26. Makeig, S. (1990) in Psychophysical Brain Research, eds. Brunia, C. H. M., Gaillard, A. W. K. & Kok, A. (Tilburg University Press, Tilburg) pp. 60-64.
27. Pantev, C., Makeig, S., Hoke, S., Galambos, R., Hampson, S. & Gallen, C. (1991) Proc Natl Acad Sci USA 88, 8996-9000.
28. Freeman, W. & Skarda, C. (1985) Brain Res Rev 10, 147-175.
29. Basar-Eroglu, C. & Basar, E. (1991) Intern J Neurosci 60, 227-237.
30. Singer, W. (1993) Ann Rev Physiol 55, 349-374.
31. Galambos, R. (1992) in Induced Rhythms in the Brain , eds. Basar, E. & Bullock, T.H. (Birkhauser, Boston), pp. 210-216.
32. Makeig, S. & Galambos, R. (1989) in Brain Dynamics: Progress and Perspectives , eds. Basar, E., and Bullock, T.H. (Springer-Verlag, Berlin), pp. 375-400.
33. Eckhorn, R., Schanze, T., Brosch, M., Salem, W., and Bauer, R. (1989) in Brain Dynamics: Progress and Perspectives , eds. Basar, E., and Bullock, T.H. (Springer-Verlag, Berlin), pp. 47-82.
34. Campbell, M. J., Lewis, D. A., Foote, S. L. & Morrison, J. H. (1987) J Comp Neurol 260, 209-220.
35. Metherate, R., Cox, C. L. & Ashe J. H. (1992) J Neurosci 12, 4701-4711.
36. Pirch, J. H., Corbus, M. J., Rigdon G. C., Lyness, W. H. (1986) Electroencephalogr clin Neurophysiol, 63, 464-75.
37. Goodin, D. S., Aminoff, M. J. & Shefrin, S. L. (1990) J Neurophysiol 64, 1270-1281.
38. Ortiz, T. A., Goodin, D. S. & Aminoff, M. J. (1993) J Neurophysiol 69, 1499-1512.
39. Kuelpe, O. (1895) Outlines of Psychology (Swan Sonneschein, London).
40. Kaufman, L., Schwartz, B., Salustri, C. & Williamson S. J. (1990) J Cognitive Neurosci 2, 124-32.
41. Kristeva-Feige, R., Feige, B., Makeig, S., Ross, B. & Elbert, T. (1993) NeuroReport , 4, 1291-94.
42. Scherg, M. & Volk, S. A. (1983) Electroencephalogr clin Neurophysiol 56, 443-452.
43. Pfurtscheller G. & Neuper C. (1992) Neuroreport 3, 1057-60.
44. Schneider, W. & Shiffrin, R. M. (1977) Psychol Rev 84, 1-66.
45. Norman, D. A. & Shallice T.(1986) in Consciousness and Self-Regulation , eds. Davidson, R. J., Schwartz, G. E. & Shapiro, D. (Plenum, New York), Vol. 4, pp. 1-18.