The EEGLAB News #2
Scott Burwell, Ph.D.
 National Institute on Drug Abuse T32 Postdoctoral Research Fellow
National Institute on Drug Abuse T32 Postdoctoral Research Fellow
Department of Psychiatry, University of Minnesota
Ph.D. Psychology, University of Minnesota
“I have many questions I’d like to answer in my lifetime,” Dr. Scott Burwell shares, “but one question that I think is especially important is to understand what position brain sensing tools using noninvasive (or invasive) electrophysiology might ultimately take in clinical practice for mental disorders.”
Dr. Burwell, a postdoctoral research fellow in the Department of Psychiatry at the University of Minnesota (UMN), has devoted much of his research to investigating methods to understand and treat addiction and related mental disorders using brain sensing tools. He is particularly interested in brain signals that may be used to predict future behavioral problems, such as unconstrained actions in adolescents or relapse in people undergoing treatment for substance use disorders. Using electroencephalographic (EEG) recording, functional magnetic resonance imaging (fMRI), and genetically-informed data analysis, his research aims to identify the brain mechanisms underlying the development and maintenance of substance abuse and addiction. “We are currently tracking brain outcomes over the course of addiction treatment using laboratory-based EEG and other physiology,” he explains. “Such brain outcomes might be useful for assessing treatment outcomes or directing treatment decisions, and / or as a form of biofeedback.”
 EEGLAB
EEGLAB
Dr. Burwell has found EEGLAB to be instrumental to his research, being an enthusiastic adopter of one of the first open-source toolboxes for comprehensive EEG signal processing. In 2008, he worked for Steve Malone at the Minnesota Center for Twin and Family Research (MCTFR) where they were looking for ways to process several-hundred newly-acquired dense array EEG and psychophysiology datasets.
“The fact that EEGLAB was freely-available, had nice visualization tools, and was implemented in a programmable environment that would facilitate automated processing on our linux computing servers made it appealing to us,” he adds. “Additionally, we found the ‘data mining’ approaches that Makeig, Delorme, and colleagues made available through EEGLAB to be fresh and exciting, and provided superior tools for modeling and analyzing EEG data relative to the proprietary (and often expensive!) software that was out there,” he admits candidly.
Currently, Dr. Burwell’s favorite features of EEGLAB include the ease of access to advanced signal processing and brain modeling tools, such as independent  component analysis (ICA) implementations, Zeynep Akalın Acar’s Neuroelectric Forward Head Modeling toolbox (NFT), and Nima Bigdely Shamlo’s Measure Projection Toolbox (MPT). Dr. Burwell has even co-developed an automated EEG preprocessing pipeline. He explains, “One way I use EEGLAB is as an integral part of the automated EEG preprocessing pipeline (github.com/sjburwell/eeg_commander) that I developed with Steve [Malone] at the Minnesota Center for Twin & Family Research (MCTFR).”
component analysis (ICA) implementations, Zeynep Akalın Acar’s Neuroelectric Forward Head Modeling toolbox (NFT), and Nima Bigdely Shamlo’s Measure Projection Toolbox (MPT). Dr. Burwell has even co-developed an automated EEG preprocessing pipeline. He explains, “One way I use EEGLAB is as an integral part of the automated EEG preprocessing pipeline (github.com/sjburwell/eeg_commander) that I developed with Steve [Malone] at the Minnesota Center for Twin & Family Research (MCTFR).”
The MCTFR is a large-scale longitudinal study of twins and their families. There, a typical EEG study often consists of several hundred EEG sessions. Dr. Burwell continues, “Large sample sizes (e.g., “big data” efforts) really favor automated EEG processing and artifact detection which are more efficient and reliable than time-intensive and subjective visual data mark-up of quality and artifacts, and the EEGLAB functions and MATLAB environment are highly useful in this way. For instance, the EEGLAB data structure and visualization tools have made it convenient to track and screen the output of our outlier-robust preprocessing pipeline that targets specific types of artifacts (e.g., bad channels, salt bridges, ocular and EMG components, etc.).”
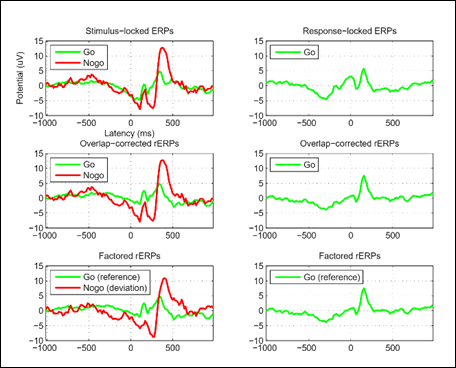 Dr. Burwell finds another huge benefit of EEGLAB – the plug-ins: “The data format ‘standard’ that EEGLAB has created has facilitated a ‘common language’ for innovative scientists to share their code with others trying to incorporate novel methods into their research. We have tested and used several of the 'plug-ins’ related to data importing, independent component classification, artifact removal, etc. Also, we have designed our in-house preprocessing and analysis software to work on EEGLAB-formatted data; e.g., my code to calculate ‘regression ERPs (rERPs)’ that we used to disentangle temporally-overlapping brain responses in our recently published paper with Scott Makeig (Burwell, Makeig, Iacono, & Malone, Psychophysiology, 2019) is available on my GitHub page and can be applied to others’ EEGLAB-formatted data.”
Dr. Burwell finds another huge benefit of EEGLAB – the plug-ins: “The data format ‘standard’ that EEGLAB has created has facilitated a ‘common language’ for innovative scientists to share their code with others trying to incorporate novel methods into their research. We have tested and used several of the 'plug-ins’ related to data importing, independent component classification, artifact removal, etc. Also, we have designed our in-house preprocessing and analysis software to work on EEGLAB-formatted data; e.g., my code to calculate ‘regression ERPs (rERPs)’ that we used to disentangle temporally-overlapping brain responses in our recently published paper with Scott Makeig (Burwell, Makeig, Iacono, & Malone, Psychophysiology, 2019) is available on my GitHub page and can be applied to others’ EEGLAB-formatted data.”
He has high hopes for the future of EEGLAB: “As EEGLAB continues to grow its support of multimodal imaging (e.g., NFT’s computational head modeling, SCALE’s skull conductivity estimation) and cloud computing (e.g., the Neuroscience Gateway Portal, OpenNeuro.org), which are both exciting and much appreciated in their own right, I am hopeful that the future of EEG+MRI multimodal integration may be moved to “plug-and-play” cloud computing resources. In my experience, full source localization of EEG within individualized subject head models acquired from MRI often require multiple software dependencies, which are not always easily configured within certain software environments. “Containerized” solutions (e.g., run using Docker or Singularity), which have gained much recent popularity in the MRI world (e.g., fmriprep, mriqc), may resolve issues surrounding software dependency requirements and replicability. However, not all researchers possess adequate supercomputing support for EEG+MRI multimodal integration, making a centralized open, cloud-based resource for multimodal integration very attractive .”
Recent Paper with Dr. Makeig
In 2012, Scott. Burwell’s path crossed that of Scott Makeig when he applied for a graduate training fellowship from the Society for Psychophysiological Research. A mentor at the University of Minnesota, Steve Malone, connected the two.
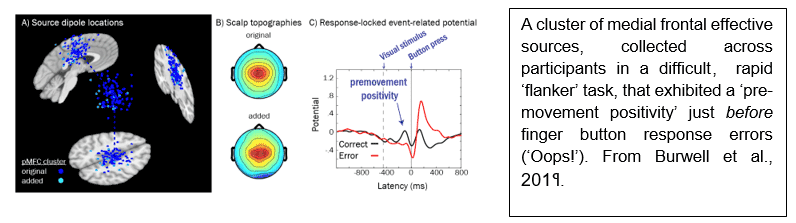
“Back then,” Dr. Burwell explains, “I was very interested in possible brain mechanisms foreshadowing human mistakes. Erroneous cognitive performance may share a common mechanism with impulsiveness and accident proneness (e.g., car crashes, etc.), which could serve as a possible preventative and therapeutic target.” He continues, “Some research in nonhuman primates suggested that firing patterns of a subset of neurons in medial frontal cortex encode the “correctness” of impending motor actions, and I wanted to see whether a comparable phenomenon might be observed in humans and might help explain an individual's proneness to making mistakes. While EEG does not sense single-neuron firing patterns, combined with independent component analysis and subject-specific source localization using MRI head images, we thought it might be possible to disentangle distinct EEG signal sources within medial frontal cortex from those from other regions of the brain, and that these distinct sources might be differentially related to correct and erroneous actions.”
This research led to the recently published article, Reduced premovement positivity during the stimulus-response interval precedes errors: Using single-trial and regression ERPs to understand performance deficits in ADHD (Burwell et al., 2019; Psychophysiology). “We found that the amplitude of the “premovement positivity,” a positive-going brain potential in or near posterior medial frontal cortex differentiated correct and erroneous actions about 200 milliseconds before the button press in a fast-paced “flankers” task. In other words, smaller amplitude of the premovement positivity signaled the likelihood of an erroneous action in the single-trial EEG preceding the action itself. We also found individual differences in the size of premovement positivity contributing to task performance; teenagers with attention-deficit hyperactivity disorder (ADHD) made more mistakes on the task and possessed smaller-amplitude premovement positivities. We think this might be a valuable biomarker for accident proneness in vulnerable people (e.g., teenagers, people with ADHD), and might have clinical utility in informing treatment or as a neuromodulation target.”
Another passion Dr. Burwell shares is his work to extend EEG technology to wearable, low-cost EEG systems (e.g., portable dry-electrode EEG headsets) so that its uses may be scaled to a variety of settings and users.
“One effort I am immersed in at the moment is focused on combining portable EEG with addiction neuroscience applications operated from a smart device, so that addiction-related brain biomarkers can be tracked possibly multiple times per day and month without patients having to visit a laboratory.”
 Dr. Burwell is excited about the rapid innovations taking place in the area of wearable and applications-grade brain sensing technology (e.g., wireless EEG headsets), and the opportunities such innovations might present for consumer and clinical applications. He explains, “Although applications-grade, wireless EEG systems often have limitations (e.g., fewer channels, greater noise susceptibility) and added challenges in modeling and localization of brain signals compared to research-grade systems, their accessibility (i.e., usability, wireless connections, dry electrodes, affordability) might facilitate translation of robust EEG “neurotechnology” into everyday life and clinical practice. For instance, brain-computer interfaces (BCI) using EEG have almost exclusively been limited to laboratory settings, but with good applications-grade EEG equipment and automated signal processing and analysis, it is growing possible to take such neurotechnology to the field, allowing for people to access their brain functions in their homes or in underserved places.”
Dr. Burwell is excited about the rapid innovations taking place in the area of wearable and applications-grade brain sensing technology (e.g., wireless EEG headsets), and the opportunities such innovations might present for consumer and clinical applications. He explains, “Although applications-grade, wireless EEG systems often have limitations (e.g., fewer channels, greater noise susceptibility) and added challenges in modeling and localization of brain signals compared to research-grade systems, their accessibility (i.e., usability, wireless connections, dry electrodes, affordability) might facilitate translation of robust EEG “neurotechnology” into everyday life and clinical practice. For instance, brain-computer interfaces (BCI) using EEG have almost exclusively been limited to laboratory settings, but with good applications-grade EEG equipment and automated signal processing and analysis, it is growing possible to take such neurotechnology to the field, allowing for people to access their brain functions in their homes or in underserved places.”
The Future
The topic of portable EEG technology naturally leads back to his initial question: What position will brain sensing tools ultimately take in clinical practice for mental disorders?
He describes some of the challenges seen in the field: “There have been brain sensing clinical tools proposed previously to diagnose disorders or prognosticate clinical outcomes (e.g., so-called ‘qEEG biomarkers’), but the evidence for these has not passed scientific rigor (e.g., replication, questionable incremental validity) and thus these remain generally unaccepted for use by health professionals (e.g., Gloss et al., 2016, AAN; Widge et al., 2019; JAMA Psychiatry). One reason I think it has been a challenging road for such ‘qEEG biomarkers’ is that the proposed tools don’t adequately model the highly complex nature of brain dynamics, relying instead on the mixed EEG signal(s) recorded at one or a few scalp electrodes.”
This is where Dr. Burwell believes EEGLAB and its plug-ins may help. “Greater adoption of advanced signal processing and source modeling approaches such as those afforded by EEGLAB and its plug-ins may improve modeling of such brain dynamics,” he states.
“Another roadblock I see as highly problematic is the current reliance on categorical diagnostic labels in psychiatry in spite of the recognized transdiagnostic and dimensional structure of mental disorders. Improvement of behavioral phenotypes through projects such as the Research Domain Criteria Initiative (Insel et al., 2010) and Hierarchical Taxonomy of Psychopathology (Kotov et al., 2015) may facilitate the mapping of brain sensing data onto clinical assessment and treatment data.”
He pauses to reflect on the huge advances in brain sensing tools and EEG analysis, and what those innovations could mean for his vision of using brain signal analysis to improve healthcare for addictions and mental disorders. He sees a bright future as he concludes, “It is an exciting time for brain and mental health innovations.”
R. Weistrop, October 2019